Page 68 - rp-may-2020
P. 68
CPD ACTIVITY 66
RET AIL PHARMA C Y • MA Y 2020
FROM PAGE 65
of children develop an immune response
against all four serogroups (A, C, W and
Y). South Australia has gone ahead and
1
funded the meningococcal B vaccine
for children aged 6 weeks to 12 months
due to the meningococcal serogroup B
disease being the predominate cause
of meningitis, with 82 per cent in SA
compared with 35 per cent in Queensland,
nine per cent in Victoria, and 29 per cent
in Western Australia.
24
Neisseria meningitidis
is normally
a commensal of the nasopharynx.
Transmission occurs via large droplet
spread or direct contact with oral
secretions. Carriage rates vary by
population worldwide and by age.
Asymptomatic carriage is most common in
adolescents and young adults.
26
Neisseria
meningitidis
has the potential to invade the
mucosa and cause invasive meningococcal
disease, which most commonly presents as
meningitis, septicaemia or both. Between 11
and 19 per cent of patients surviving
invasive meningococcal disease have
complications, with higher complication
rates in children compared with adults.
Complications range widely and may
develop as physical, neurologic and
psychiatric sequelae. In 2014–2016, twice
as many meningococcal hospitalisations
were among indigenous compared with
non-indigenous Australians.
27
Hepatis A
The hepatitis A vaccination program was
initially established in North Queensland
in 1999 for Aboriginal and Torres Strait
Islander children aged 18 months. In 2005,
it expanded to include all Aboriginal
and Torres Strait Islander children aged
12 months in the Northern Territory,
Queensland, South Australia and Western
Australia. The hepatitis A vaccine is
1
an inactivated whole virus vaccine.
The contraindications and adverse effects
are reported in Table 1.
Before the vaccination program, rates
of hepatitis A in Aboriginal and Torres
Strait Islander communities were very
high. Factors associated with high rates
were poor living conditions, overcrowding
and poor sanitation. The hepatitis A
16
vaccination program for Aboriginal
and Torres Strait Islander children in
endemic areas substantially reduced
hospitalisations and notifications for
this population.
1
Conclusion
The number of vaccines administered
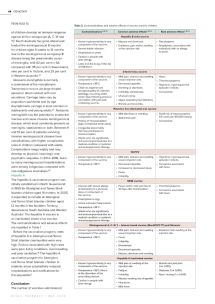
Table 2.
Contraindications and adverse effects of vaccine used for children.
Contraindications
1, 30, 31
Common adverse effects
1, 30, 31
Rare adverse effects
1, 30, 31
Hepatitis B child vaccine
• Known hypersensitivity to any
component of the vaccine.
• Severe febrile infection.
• Anaphylaxis to yeast.
• Caution in people with
latex allergy.
• Latex is in the bung of the vial
of H-B-Vax II.
• Nausea and diarrhoea.
• Erythema, pain and/or swelling
at the injection site.
• Pancytopenia.
• Anaphylaxis, associated with
individuals with an allergy
to yeast.
Infanrix-hexa vaccine
• Known hypersensitivity to any
component of the vaccine.
• Temperature >38C.
• Child has experienced
encephalopathy of unknown
aetiology, occurring within
seven days following previous
vaccination with pertussis
containing vaccine.
• Mild pain, redness and swelling
around injection site.
• Decreased appetite.
• Vomiting or diarrhoea.
• Irritability, restlessness.
• Unusual crying.
• Upper respiratory tract infections.
• Rhinitis and bronchitis.
• Hives.
• Thrombocytopenia.
• Hypotonic, hyporesponsive
episode in infants.
• Convulsions.
Rotavirus live attenuated oral vaccine
• Known hypersensitivity to any
component of the vaccine.
• History of intussusception
(type of intestinal obstruction).
• Congenital abnormality
that may predispose to
intussusception.
• Infants who are significantly
immunocompromised due to a
medical condition or systemic
immunosuppressive therapy.
• Mild diarrhoea or vomiting.
• Abdominal pain.
• Small risk of intussusception
(1.5 cases per 100,000 doses).
• Convulsions.
13cPCV
• Known hypersensitivity to any
component of the vaccine.
• Temperature >38C.
• Mild pain, redness and swelling
around injection site.
• Decreased appetite.
• Increased or decreased sleep.
• Fever.
• Irritability.
• Hypotonic, hyporesponsive
episode in infants.
• Convulsion associated
with fever.
MMR vaccine
• Anyone with severe allergy
(anaphylaxis) to a previous
dose or component of
the vaccine.
• Anaphylaxis to egg.
• Active untreated tuberculosis.
• Temperature >38C.
• Infants who are significantly
immunocompromised due to a
medical condition or systemic
immunosuppressive therapy.
• Fever and/or mild rash five to
12 days after immunisation.
• Thrombocytopenia.
• Convulsion associated
with fever.
Meningococcal A, C, W Y – tetanus toxoid vaccine (MenACWY-TT) and Nimenrix
• Known hypersensitivity to any
component of the vaccine.
• Temperature >38C.
• Mild pain, redness and swelling
around injection site.
• Fever.
• Irritability.
• Drowsiness.
• Decreased appetite.
• Nausea, diarrhoea and vomiting.
• Extensive limb swelling at the
injection site.
Hepatitis A inactivated vaccine
• Known hypersensitivity to any
component of the vaccine.
• Temperature >38C (this is
at the discretion of the
prescribing doctor).
• Caution in people with
latex allergy.
• Mild pain or swelling at the
injection site.
• Fatigue.
• Irritability.
• Nausea, vomiting, loss of appetite.
• Headache.
• Mild fever.
• Muscle and joint pain
(1 in 1,000).
• Dizziness (1 in 1,000).
• Rash / itching (1 in 1,000).