Page 3 - rp-may-2020
P. 3
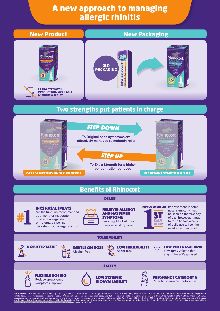
A new approach to managing
allergic rhinitis
New Packaging
New Product
OLD
PACKAGING
STEP DOWN
STEP UP
To Original once symptoms are
effectively managed to maintain relief
To Extra Strength for a higher
concentration per dose
GETS SYMPTOMS UNDER CONTROL
MAINTAINS SYMPTOM RELIEF
Two strengths put patients in charge
EXTRA STRENGTH
PRODUCT NOW AVAILABLE
WITHOUT A SCRIPT
Benefits of Rhinocort
INCS nasal sprays
are the first-line recommended
treatment for moderate
to severe allergic rhinitis
symptoms, as well as
persistent mild allergic rhinitis.
Relieves allergy
and hayfever
symptoms
Sneezing, blocked
nose,
runny nose, itchy nose.
Gentle on nose
Alcohol-free
Low irritability
Scent-free
Improvement in some
nasal symptoms may
be seen on the first day
of use, however, it may
take up to two weeks
of daily use to feel the
most symptom relief.
RELIEF
TOLERABILITY
SAFETY
No bitter taste
2–4
Low post nasal drip
Low spray volume that
stays where it's sprayed
Low systemic
bioavailability
Flexible dosing
Reduce sprays once
symptoms improve
Pregnancy Category A
5
Refer to pharmacist before use
IMPROVEMENT ON
St
DAY
1OF USE
References: 1.
Seidman MD et al. Clinical practice guideline: Allergic rhinitis. Otolaryngol Head Neck Surg. 2015 Feb; 152(1 Suppl): S1–43.
2.
Shah SR et al. Two multicenter, randomized, single-blind,
single-dose, crossover studies of specific sensory attributes of budesonide aqueous nasal spray and fluticasone propionate nasal spray. Clin Ther. Clin Ther. 2003 Aug; 25(8): 2198–214. Khanna P, Shah A.
3.
Assessment of sensory perceptions and patient preference for intranasal corticosteroid sprays in allergic rhinitis. Am J Rhinol 2005; 19(3): 316–321. Meltzer EO. Formulation considerations of intranasal
4.
corticosteroids for the treatment of allergic rhinitis. Ann Allergy Asthma Immunol. 2007 Jan; 98(1): 12–21. Therapeutic Goods Administration Prescribing Medicines in Pregnancy Database. Available at
5.
www.tga.gov.au/prescribing-medicines-pregnancydatabase(Accessed April 2020).